Difference between revisions of "Urothelium"
(→IHC) |
|||
| Line 538: | Line 538: | ||
===IHC=== | ===IHC=== | ||
*Ki-67: | *Ki-67: | ||
**Rajcani et al.:<ref name=pmid23944616>{{Cite journal | last1 = Rajcani | first1 = J. | last2 = Kajo | first2 = K. | last3 = Adamkov | first3 = M. | last4 = Moravekova | first4 = E. | last5 = Lauko | first5 = L. | last6 = Felcanova | first6 = D. | last7 = Bencat | first7 = M. | title = Immunohistochemical characterization of urothelial carcinoma. | journal = Bratisl Lek Listy | volume = 114 | issue = 8 | pages = 431-8 | month = | year = 2013 | doi = | PMID = 23944616 }}</ref> <25% of tumour cells for low-grade versus >50% tumour cell for high-grade. | **Rajcani ''et al.'':<ref name=pmid23944616>{{Cite journal | last1 = Rajcani | first1 = J. | last2 = Kajo | first2 = K. | last3 = Adamkov | first3 = M. | last4 = Moravekova | first4 = E. | last5 = Lauko | first5 = L. | last6 = Felcanova | first6 = D. | last7 = Bencat | first7 = M. | title = Immunohistochemical characterization of urothelial carcinoma. | journal = Bratisl Lek Listy | volume = 114 | issue = 8 | pages = 431-8 | month = | year = 2013 | doi = | PMID = 23944616 }}</ref> <25% of tumour cells for low-grade versus >50% tumour cell for high-grade. | ||
**Pich et al.:<ref>{{Cite journal | last1 = Pich | first1 = A. | last2 = Chiusa | first2 = L. | last3 = Comino | first3 = A. | last4 = Navone | first4 = R. | title = Cell proliferation indices, morphometry and DNA flow cytometry provide objective criteria for distinguishing low and high grade bladder carcinomas. | journal = Virchows Arch | volume = 424 | issue = 2 | pages = 143-8 | month = | year = 1994 | doi = | PMID = 7910097 }}</ref> 11%/17% G1/G2 versus 34% G3. | **Pich ''et al.'':<ref name=pmid7910097>{{Cite journal | last1 = Pich | first1 = A. | last2 = Chiusa | first2 = L. | last3 = Comino | first3 = A. | last4 = Navone | first4 = R. | title = Cell proliferation indices, morphometry and DNA flow cytometry provide objective criteria for distinguishing low and high grade bladder carcinomas. | journal = Virchows Arch | volume = 424 | issue = 2 | pages = 143-8 | month = | year = 1994 | doi = | PMID = 7910097 }}</ref> 11%/17% for G1/G2 versus 34% for G3. | ||
===Molecular=== | ===Molecular=== | ||
Revision as of 12:42, 17 October 2013
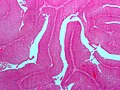
The urothelium lines the upper portion of the genitourinary tract, i.e. ureters, urinary bladder), and a bit of the lower part.
Normal urothelium
Gross
Extent of urothelium
- Ureters.
- Renal pelvis.
- Urinary bladder.
- Part of the urethra.
Urethra in males
- Pre-prostatic urethra - transitional epithelium.
- Prostatic urethra - transitional epithelium.
- Membranous urethra (from apex of prostate to bulb of penis (bulb of the corpus spongiosusm)) - pseudostratified columnar epithelium.
- Spongy urethra - pseudostratified columnar epithelium (proximal) & stratified squamous (distal).
Microscopic
Features:
- Maturation (cuboidal at base - squamoid at surface).
- Surface cells called 'umbrella cells' (umbrella cells CK20 +ve).
- Urothelium should be 4-5 cell layers thick.
- +/-Prominent nucleoli.
Note:
- Should not have a papillary architecture -- if it does it is likely cancer!
- If it is 'papillary' -- it must have fibrovascular cores.
Sign out
URINARY BLADDER LESION, TRANSURETHRAL RESECTION: - UROTHELIAL MUCOSA WITHIN NORMAL LIMITS. - NEGATIVE FOR MALIGNANCY.
Micro
The sections shows urothelium with underlying tissue. The urothelium is 4-5 cells thick. Umbrella cells are present. Few mononuclear inflammatory cells are seen in the subepithelial tissue.
The urothelium has no nuclear hyperchromasia and no significant nuclear enlargement. Mitotic activity is not identified. No papillary structures are present.
Approach
Where to start
July 1st PGY-2:
- Urothelial carcinoma - essentially defined by increased nuclear size +/- irreg. nuclear contour.
- Nucleoli are common in urothelium.
- This can be confusing... prostate carcinoma has nucleoli.
- Mitosis - these are key if the nuclear enlargement is not present.[1]
- Cell-depleted urothelium, where the cells have shed-off--but a few remain, should raise suspicions to cancer.
- Thickness of the urothelium, otherwise, isn't very useful for diagnosing cancer.
- Nucleoli are common in urothelium.
- Round structures should make you think of papillae and prompt looking for fibrovascular cores.
- Fibrovascular cores = papillae... may be cancer!
A checklist-like approach
- Papillary structure - with fibrovascular cores?
- Nuclear pleomorphism?
- Yes - high grade (4-5x lymphocyte) --> Dx: high grade papillary urothelial carcinoma
- No - low grade or normal (2-3x lymphocyte) --> DDx: low grade papillary urothelial carcinoma, PUNLMP, papilloma
- Nuclear pleomorphism?
- Flat lesions?
- Nuclear pleomorphism?
- Maturation to surface?
- No --> Dx: sectioning artefact vs. flat UCC.
- Yes --> likely benign.
- Normal thickness?
- Normal is 4-5 cell layers.
- Nests of glandular cells
- Consider cystitis cystica, cystitis glandularis, cystitis cystica et glandularis, von Brunn's nest, inverted papilloma.
- Inflammation?
- Michaelis-Gutman bodies?
Pitfalls:
- Urothelial carcinoma of the bladder may be confused with a paraganglioma of the bladder.
- Way to differentiate: paraganglioma = stippled chromatin, UCC = single nucleoli.
Note about terminology
- The bladder is rather unique in that "carcinoma" is a label used for things that are non-invasive.
- It has been suggested that many things that are called papillary urothelial carcinoma, would be better described as papillary intraurothelial neoplasia.[2]
- If the terminology in the urinary bladder were applied to the colon, we'd call all adenomas, i.e. pre-malignant lesions, carcinomas.
Overview in tables
General categorization
Urothelial lesions can broadly be divided into:
- Flat lesions.
- Lack papillae.
- Tend to be more aggressive.
- Papillary lesions.
- Must have true papillae.
- Very common.
- More often benign/indolent.
Flat urothelial lesions
Comparison urothelial changes - flat epithelium - benign/premalignant/cancerous:[3]
| Diagnosis | Nuclear enlargement (X stromal lymphocyte) |
Nucleoli | size var., shape | Polarity | Mitoses | Thickness | Inflammation | Other |
|---|---|---|---|---|---|---|---|---|
| Normal | none (2x) | small | none, round | matures to surface | none/minimal | 4-5 cells | none | - |
| Reactive atypia | moderate, prominent (3x) | prominent | none, round | as normal | some, none atypical | as normal | severe, acute or chronic | - |
| Flat urothelial hyperplasia | none (2x) | small | none, round | as normal | as normal | increased | usu. none | - |
| Urothelial dysplasia | moderate (3x) | small, some multiple | mod. variation, some irregularity | lost | rare, none atypical | as normal | usu. none | - |
| UCC in situ | signif. (4-5x) | +/-large | marked, irregular | lost | common, atypical | thin, thick or norm. | +/- | - |
| Invasive UCC | signif. (4-5X) | +/-large | marked, irregular | lost | common, atypical | thin, thick or norm. | +/- | stromal invasion |
The bold entry is considered the key feature.
Papillary urothelial lesions
Urothelial cells in papillae - benign/premalignant/cancerous:[4][5]
| Diagnosis | Papillae features | Papillae branching | Papillae fusion | Nuclear size | Mitoses | DDx | IHC | Other | Key feature |
|---|---|---|---|---|---|---|---|---|---|
| Papilloma | fat papillae, thick FV core |
rare | none | normal (2x lymphocyte) | very rare basal | PUNLMP, low gr. PUCC | p53-, CK20+ umbrella cells | cytologically normal | normal cells, fat papillae |
| PUNLMP | slender FV core | uncommon | rare | enlarged - uniform | rare basal only | papilloma, low gr. | CK20+ umbrella | low cellular density (@ low power) vs. low gr.[6] | uniformly enlarged cell pop., slender papillae |
| Low grade PUCC | slender FV core, thick epithelium |
frequent | some | enlarged with variation | infreq., usually basal | PUNLMP, high gr. | -/+ p53, CK20+ umbrella | +/- small nucleoli | nuc. pleomorphism, thick epithelium |
| High grade PUCC | mixed population | common | common | 4-5x lymphocyte, marked pleomorphism |
common, everywhere | low gr., invasive UCC | diffuse CK20+, p53+ in 50% | nucleoli prominent | marked nuclear pleomorphism |
Notes:
- FV core = fibrovascular core.
- PUCC = papillary urothelial carcinoma.
Risk factors for urothelial carcinoma
Others:
- Lynch syndrome.
- Should be considered in ureteral cancers.[9]
Flat urothelial lesions
Overview
Several different benign & pre-malignant diagnoses can be made.
The World Health Organization classification is:[10]
- Reactive urothelial atypia.
- Flat urothelial hyperplasia.
- Urothelial atypia of unknown significance.
- Urothelial dysplasia (low-grade dysplasia).
- Urothelial carcinoma in situ (high-grade dysplasia).
- Invasive urothelial carcinoma.
Mild urothelial atypia in normal urothelium
General
- May be confused with urothelial carcinoma in situ.[11]
- Uncommon.
Microscopic
Features:[11]
- Umbrella cells have:
- Mild nuclear enlargement ~3-4x lymphocyte.
- Round/regular nuclear membranes.
- Focally clear cytoplasm with cobwebs.
- Clear cytoplasm with eosinophilic reticulations.
- +/-Inflammation.
- No mitotic activity.
DDx:
IHC
- Ki-67 low.
- p53 -ve.
Urothelial carcinoma in situ
- Abbreviated CIS.
General
- Lack papillae.
Microscopic
Features:
- Nuclear changes key feature.
- Enlargement of nuclei (often 4-5x the size of stromal lymphocytes) -- diagnostic.[12]
- Normal urothelium approx. 2x the size of stromal lymphocytes.
- Nuclear pleomorphism - marked variation in size of nuclei.
- Enlargement of nuclei (often 4-5x the size of stromal lymphocytes) -- diagnostic.[12]
- +/-Disordered arrangement/crowding of cells.
- In normal urothelium the cell line-up on the basement membrane.
- Umbrella cells often absent.
- +/-Mitoses present.
- +/-Enlarged nucleoli.
Note:
- The urothelium may be "depleted", i.e. exist only of rare large cells on the basement membrane.
- This is known as clinging urothelial carcinoma in situ.[13]
IHC
Features:[14]
- p53 +ve.
- Ki-67 high.
Benign urothelium vs. CIS:[15]
- CK20 +ve in deep cells (23/26 cases).
- Normal urothelium -- only the umbrella cells.
- Ki-67 ~50% of cells - deep and superficial.
- Normal ~10% of cells, confined to basal aspect.
Sign out
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION URINARY BLADDER TUMOUR (TURBT):
- UROTHELIAL CARCINOMA IN SITU.
- MUSCULARIS PROPRIA PRESENT.
Urothelial cell carcinoma
- See urine cytology for the cytopathology.
- Abbreviated UCC.
- AKA urothelial carcinoma.
General
- These lesions lack papillae and are typical flat.
- Clinically, it may not be possible to differentiate renal pelvis urothelial carcinoma and renal cell carcinoma.
Microscopic
Features:
- Nuclear pleomorphism - key feature.
- Compare nuclei to one another.
- Increased N/C ratio.
- Lack of maturation to surface (important).
- Cells become dyscohesive.
- Mostly useless in my experience.
Invasion vs. in situ: Useful features - present in invasion:[16]
- Thin-walled vessels.
- Stromal reaction (hypercellularity).
- Retraction artefact around the tumour cell nests.
Note:
- The presence/absence of muscle should be commented on in biopsy specimens.
- Adipose tissue may be seen in the lamina propria; tumour adjacent to adipose tissue on a biopsy does not imply invasion deep to the muscularis propria.[17]
Staging
- T1 - lamina propria.
- Several subdivisions of T1 exist:
- T1a - superficial or in muscularis mucosae.
- T1b - beyond muscularis mucosae - into submucosa.
- Several subdivisions of T1 exist:
- T2 - muscularis propria.
Subtypes of urothelial carcinoma
There are numerous subtypes:[18]
- Squamous differentiation.
- Clear cell.
- Plasmacytoid.
- Micropapillary.
- Small nests (< ~10 cells/nest).
- Sarcomatoid.
- Many others...
Benign patterns - mnemonic Much GIN:
- Microcystic.
- Small tubular/glandular.
- Inverted.
- Nested.
Plasmacytoid urothelial cell carcinoma
Features:
- Abundant gray cytoplasm, eccentric nucleus.
Images:
Nested urothelial cell carcinoma
- AKA nested variant urothelial cell carcinoma.
Features:[19]
- High density of well-circumscribed nests.
- Mild-to-moderate nuclear atypia.
- +/-Foci of unequivocal conventional urothelial carcinoma.
- Focally solid or gland fusion.
- Moderate-to-severe nuclear atypia +/- abundant mitoses.
- +/-Extension into the muscularis propria.
DDx:
Images
www:
IHC
Features:
- CK7 +ve CK20 +ve.
- CK20 may be negative.
UCC vs. Prostate:
- UCC: p63+, PSA-, PSAP-, CK7+, CK20+.
- Prostate: p63-, PSA+, PSAP+, CK7-, CK20-.
UCC vs. RCC:
- UCC: p63+.[21]
Molecular
Not used for diagnosis.
Changes:
- 9p deletion -- site of CDKN2A[22] (AKA p16).
- 17p deletion -- site of PT53 (AKA p53).
Sign out
High grade UCC
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION URINARY BLADDER TUMOUR (TURBT):
- INVASIVE HIGH-GRADE PAPILLARY UROTHELIAL CARCINOMA WITH SQUAMOUS DIFFERENTIATION AT LEAST INTO MUSCULARIS PROPRIA.
- LYMPHOVASCULAR INVASION PRESENT.
Nested variant
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION OF BLADDER TUMOUR (TURBT):
- INVASIVE LOW-GRADE UROTHELIAL CARCINOMA, NESTED VARIANT.
- TUMOUR PRESENT AT EDGE OF TISSUE.
- NO MUSCULARIS PROPRIA IDENTIFIED.
Papillary urothelial lesions
Papillary urothelial lesions are grouped into one of five categories (listed from good to bad prognosis):[5]
- Urothelial papilloma.
- Inverted papilloma.
- Papillary urothelial neoplasm of low malignant potential (PUNLMP).
- PUNLMP is pronouced "pun-lump".
- Low grade papillary urothelial carcinoma.
- High grade papillary urothelial carcinoma.
Key characteristics:
- Nuclear - size/pleomorphism.
- Papillae branching.
- Papillae fusion.
Urothelial papilloma
General
- Very rare diagnosed.
- If the person has a history of a low grade papillary urothelial carcinoma... it is a low grade papillary urothelial carcinoma.
- These cases are a consensus diagnosis, i.e. you show it to a colleague... if they agree you can call it.
Microscopic
Features:[5]
- Papillary fronds.
- Minimal branching or fusion.
- Cytological features of normal urothelium.
- Normal urothelium approx. 2x the size of stromal lymphocytes.[12]
- No mitoses.
- Thickness < 7 cells.[citation needed]
DDx:
Inverted urothelial papilloma
General
- May be confused with papillary urothelial carcinoma with an inverted growth pattern.
Microscopic
Features:
- Like papillomas... but grow downward.[5]
- According to THvdK,[23] inverted papillomas never have an exophytic component; if an exophytic component is present it is urothelial carcinoma. This is disputed by one paper from Mexico that examines two cases.[24]
- Nests have peripheral palisading of nuclei - important.
DDx:
- Low grade papillary urothelial carcinoma with an inverted growth pattern.
Images
Papillary urothelial neoplasm of low malignant potential
- Abbreviated PUNLMP.
General
- Uncommon: prevalence ~ 0-3.5%.[25]
- PUNLMP vs. low grade papillary urothelial carcinoma has a poor inter-rater reliability.[26]
Treatment:
- Excision and on-going follow-up - like non-invasive low grade papillary urothelial carcinoma (LGPUC).[27]
Microscopic
Features:[5]
- Rare fused papillae.
- Infrequent mitoses.
- Nuclei larger than papilloma - but monotonous.[29]
DDx:
Images
Low grade papillary urothelial carcinoma
General
- Very common.
- Very good prognosis - if it is non-invasive.
Microscopic
Features:[5]
- Fused papillae.
- Papillae branch.
- Larger nuclei than PUNLMPs.
- +/-Invasion into the lamina propria.
Note:
- The presence/absence of muscle should be commented on in biopsy specimens.
- Adipose tissue may be seen in the lamina propria; tumour adjacent to adipose tissue on a biopsy does not imply invasion deep to the muscularis propria.[17]
DDx:
- PUNLMP.
- High grade papillary urothelial carcinoma.
- Inverted urothelial papilloma - often have peripheral palisading.
- Urothelial papilloma.
IHC
- Ki-67:
Molecular
Molecular changes:[35]
- FGFR3
- HRAS
- Loss of heterozygosity - chromosome 9.
Note:
- Not currently used diagnostically.
Sign out
URINARY BLADDER LESION ("TUMOUR"), RESECTION:
- LOW-GRADE PAPILLARY UROTHELIAL CARCINOMA.
-- NEGATIVE FOR LAMINA PROPRIA INVASION.
- NO MUSCULARIS PROPRIA IDENTIFIED.
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION OF BLADDER TUMOUR (TURBT):
- LOW-GRADE PAPILLARY UROTHELIAL CARCINOMA.
- NEGATIVE FOR LAMINA PROPRIA INVASION.
- NO MUSCULARIS PROPRIA IDENTIFIED.
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION OF BLADDER TUMOUR (TURBT):
- LOW-GRADE PAPILLARY UROTHELIAL CARCINOMA.
- NEGATIVE FOR LAMINA PROPRIA INVASION.
- MUSCULARIS PROPRIA PRESENT.
High grade papillary urothelial carcinoma
- Abbreviated HGPUC.
- AKA high grade papillary urothelial cell carcinoma, abbreviated HGPUCC.
General
- Aggressive.
Microscopic
Features:[5]
- "High grade nuclear features":
- Nuclear pleomorphism - often 4-5x the size of stromal lymphocytes.[12]
- Architectural complexity.
- Fused papillary common.
- Papillae branch.
- Mitoses common.
- +/-Invasion into the lamina propria.
Note:
- The presence/absence of muscle should be commented on in biopsy specimens.
- Adipose tissue may be seen in the lamina propria; tumour adjacent to adipose tissue on a biopsy does not imply invasion deep to the muscularis propria.[17]
DDx:
IHC
- Ki-67 >50% of tumour cells.[33]
- Low-grade UCC <25% of tumour cells positive.
Molecular
Molecular changes:[35]
- p53.
- p21.
- RB.
- E-cadherin - decreased bad.
- RhoGD12 - increased bad.
- VEGF - increased bad.
Sign out
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION:
- HIGH-GRADE PAPILLARY UROTHELIAL CARCINOMA.
- NO LAMINA PROPRIA INVASION APPARENT.
- NEGATIVE FOR LYMPHOVASCULAR INVASION.
- NO MUSCULARIS PROPRIA IDENTIFIED.
Invasion into the lamina propria
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION URINARY BLADDER TUMOUR (TURBT):
- INVASIVE HIGH-GRADE PAPILLARY UROTHELIAL CARCINOMA WITH LAMINA PROPRIA INVASION.
- MUSCULARIS PROPRIA NEGATIVE FOR INVASIVE MALIGNANCY.
- NEGATIVE FOR LYMPHOVASCULAR INVASION.
Invasion into the muscularis propria
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION URINARY BLADDER TUMOUR (TURBT):
- INVASIVE HIGH-GRADE PAPILLARY UROTHELIAL CARCINOMA AT LEAST INTO MUSCULARIS PROPRIA.
- LYMPHOVASCULAR INVASION PRESENT.
Low-grade versus high-grade
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION URINARY BLADDER TUMOUR (TURBT):
- HIGH-GRADE PAPILLARY UROTHELIAL CARCINOMA, SEE COMMENT.
- NEGATIVE FOR LAMINA PROPRIA INVASION.
- NO MUSCULARIS PROPRIA PRESENT.
COMMENT:
The sections show papillary branching, papillary fusion and scattered large cells (~4-5 a
resting lymphocyte). Atypical for a high-grade lesion is that mitotic activity is scarce
and prominent nucleoli are not present.
Micro
The sections show a small fragment of urothelial mucosa with two papillary structures, enlarged nuclei (~3-4x resting lymphocyte) and moderate nuclear size variation. Mitotic activity is seen focally. Umbrella cells are seen only focally.
A mild lymphocyte-predominant inflammatory infiltrate is present. The lamina propria contains a nest with smaller cells, cystic spaces and no appreciable mitoses (cystitis cystica).
Benign urothelial lesions
The big table of cystitis:
| Type | Key feature | DDx | Reference |
|---|---|---|---|
| Florid proliferative cystitis | expanded lamina propria with von Brunn's nests, cystitis cystica et glandularis | von Brunn's nests, cystitis cystica et glandularis, low-grade urothelial carcinoma | [36] |
| Polypoid cystitis | wide base, height > base | papillary cystitis, bullous cystitis | [37] |
| Bullous cystitis | wide base, height < base | papillary cystitis, polypoid cystitis | [37] |
| Papillary cystitis | narrow base, height > base | polypoid cystitis, bullous cystitis | [37] |
| Interstitial cystitis | +/-ulceration (uncommon) - requires clinical correlation | urothelial CIS | [38] |
| Follicular cystitis | lymphoid follicles | non-Hodgkin lymphoma | [39] |
| Infectious cystitis | dependent cause (bacterial, viral, fungal) | [40] | |
| Granulomatous cystitis | granulomas | tuberculosis, schistosomiasis, fungal infection, post-BCG | [40] |
| Radiation cystitis | edema, vascular congestion, +/- erosions -- acute; fibrosis in LP and detrusor -- chronic | [41] |
Interstitial cystitis
General
- Chronic cystitis, culture negative.
- Treatment difficult.[42]
Epidemiology:[43]
- Women > men.
Symptoms:[43]
- Urgency.
- Frequency.
- Pain.
Microscopic
Features:[38]
- +/-Ulceration (uncommon).
Note:
- Diagnosis requires clinical correlation.
DDx:
- Urothelial CIS.
Follicular cystitis
Microscopic
Features:[39]
- Lymphoid follicles in the lamina propria.
DDx:
- Non-Hodgkin lymphoma.
Sign out
URINARY BLADDER, BIOPSY: - UROTHELIAL MUCOSA WITH CHRONIC INFLAMMATION AND BENIGN LYMPHOID NODULES WITH GERMINAL CENTRE FORMATION. - MUSCULARIS PROPRIA PRESENT. - NEGATIVE FOR UROTHELIAL CARCINOMA IN SITU AND NEGATIVE FOR MALIGNANCY.
Polypoid cystitis
General
- Uncommon.
- Wide age range.
- Benign.
Microscopic
Features:[37]
- Polypoid urothelium-covered projections with:
- Broad bases.
- Height > base.
- Extensive edema.
DDx:
- Papillary cystitis - not a broad base.
- Bullous cystitis.
Image:
von Brunn nests
General
- Benign.
Microscopic
Features:[44]
- Nests of (benign) urothelium budding into the lamina propria.
Note:
- Nests should not extend into the muscularis propria.
DDx:
- Nested urothelial cell carcinoma.[45]
- Inverted papilloma.
- Cystitis cystica - have lumens, may be focal.
IHC
Features:[45]
- p53 -ve.
- MIB-1 <3%.
Cystitis cystica
General
- Benign.
- Can be thought of as von Brunn nests with cystic change.[46]
- Called ureteritis cystica if it happens in a ureter.
Microscopic
Features:[44]
- Nests of urothelium within the lamina propria with cyst formation, i.e. lumens are present.
Note:
- Nests should not extend into the muscularis propria.
DDx:
Image:
Sign out
URINARY BLADDER, BIOPSY: - CYSTITIS CYSTICA. - NEGATIVE FOR MALIGNANCY.
Cystitis glandularis
| Cystitis cystica et glandularis | |
|---|---|
| External resources | |
| EHVSC | 10173 |
- Cystitis cystica et glandularis redirects to here.
General
- Benign.
- Can be thought of as cystitis cystica with mucin-secreting cells lining the cystic spaces.[46]
- When seen in conjunction with cystitis cystica it is called cystitis cystica et glandularis.
Note:
Microscopic
Features:[44]
- Nests of urothelium within the lamina propria with cyst formation, i.e. lumens are present.
- Cyst lining cells are cuboidal and/or columnar epithelium.
- Produce mucin.
- +/-Goblet cells, i.e. intestinal metaplasia.[46]
Note:
- Nests should not extend into the muscularis propria.
Image:
Sign out
URINARY BLADDER NECK, BIOPSY: - CYSTITIS CYSTICA ET GLANDULARIS. - NEGATIVE FOR MALIGNANCY.
Micro
The sections show urothelial mucosa with bland nests within the lamina propria with cyst formation. The stroma is edematous and has a mixed inflammatory infiltrate consisting of plasma cells, eosinophils, lymphocytes and neutrophils.
Malakoplakia
Nephrogenic adenoma
General
Features:[51]
- Benign.
- May mimic adenocarcinoma!
- Classic location is the urinary bladder.
- Also reported in ureter and prostatic urethra.
- It is thought to result from displacement of renal tubular cells, as this entity in renal transplant recipients is graft derived.[52]
Microscopic
Features:[51]
- Tubular structures - key feature.
- Hobnailed cells.
- +/-Thick eosinophilic basement membrane.
- Microcystic appearance.
- Usually associated with chronic inflammation.
Notes:
- May mimic vascular/lymphatic channels - can be sorted-out with IHC.
DDx:
- Urothelial carcinoma, microcystic and nested variants.
- Prostatic adenocarcinoma.
- Clear cell adenocarcinoma.
Images
www:
IHC
Features:[54]
- CK7 +ve.
- PAX2 +ve.
- PAX8 +ve.
- AMACR +ve/-ve.
Others:[51]
- p53 -ve.
- CEA -ve.
- Ki-67 low (<5%).
See also
References
- ↑ JS. 9 June 2010.
- ↑ Van der Kwast, TH.; Zlotta, AR.; Fleshner, N.; Jewett, M.; Lopez-Beltran, A.; Montironi, R. (Dec 2008). "Thirty-five years of noninvasive bladder carcinoma: a plea for the use of papillary intraurothelial neoplasia as new terminology.". Anal Quant Cytol Histol 30 (6): 309-15. PMID 19160695.
- ↑ Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 155-163. ISBN 978-0443066771.
- ↑ Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 166-175. ISBN 978-0443066771.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 310. ISBN 978-0781765275.
- ↑ GAG. 26 February 2009.
- ↑ Chacko, JA.; Heiner, JG.; Siu, W.; Macy, M.; Terris, MK. (Jan 2006). "Association between marijuana use and transitional cell carcinoma.". Urology 67 (1): 100-4. doi:10.1016/j.urology.2005.07.005. PMID 16413342.
- ↑ URL: http://content.nejm.org/cgi/content/full/343/17/1268. Accessed on: 27 May 2010.
- ↑ Crockett, DG.; Wagner, DG.; Holmäng, S.; Johansson, SL.; Lynch, HT. (May 2011). "Upper urinary tract carcinoma in Lynch syndrome cases.". J Urol 185 (5): 1627-30. doi:10.1016/j.juro.2010.12.102. PMID 21419447.
- ↑ Hodges, KB.; Lopez-Beltran, A.; Davidson, DD.; Montironi, R.; Cheng, L. (Feb 2010). "Urothelial dysplasia and other flat lesions of the urinary bladder: clinicopathologic and molecular features.". Hum Pathol 41 (2): 155-62. doi:10.1016/j.humpath.2009.07.002. PMID 19762067.
- ↑ 11.0 11.1 Amin, Mahul B. (2010). Diagnostic Pathology: Genitourinary (1st ed.). Amirsys. pp. 2-57. ISBN 978-1931884280.
- ↑ 12.0 12.1 12.2 Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 161. ISBN 978-0443066771.
- ↑ Amin, Mahul B. (2010). Diagnostic Pathology: Genitourinary (1st ed.). Amirsys. pp. 2-55. ISBN 978-1931884280.
- ↑ Lopez-Beltran, A.; Jimenez, RE.; Montironi, R.; Patriarca, C.; Blanca, A.; Menendez, CL.; Algaba, F.; Cheng, L. (Nov 2011). "Flat urothelial carcinoma in situ of the bladder with glandular differentiation.". Hum Pathol 42 (11): 1653-9. doi:10.1016/j.humpath.2010.12.024. PMID 21531007.
- ↑ Yin, H.; He, Q.; Li, T.; Leong, AS. (Sep 2006). "Cytokeratin 20 and Ki-67 to distinguish carcinoma in situ from flat non-neoplastic urothelium.". Appl Immunohistochem Mol Morphol 14 (3): 260-5. PMID 16932015.
- ↑ Sternberg, SE. Histology for Pathologists. P.2047.
- ↑ 17.0 17.1 17.2 Bochner, BH.; Nichols, PW.; Skinner, DG. (Mar 1995). "Overstaging of transitional cell carcinoma: clinical significance of lamina propria fat within the urinary bladder.". Urology 45 (3): 528-31. doi:10.1016/S0090-4295(99)80030-2. PMID 7879346.
- ↑ URL: http://www.nature.com/modpathol/journal/v22/n2s/full/modpathol200926a.html. Accessed on: 19 August 2011.
- ↑ Talbert, ML.; Young, RH. (May 1989). "Carcinomas of the urinary bladder with deceptively benign-appearing foci. A report of three cases.". Am J Surg Pathol 13 (5): 374-81. PMID 2712189.
- ↑ Terada, T. (Oct 2011). "Nested variant of urothelial carcinoma of the urinary bladder.". Rare Tumors 3 (4): e42. doi:10.4081/rt.2011.e42. PMC 3282447. PMID 22355497. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3282447/.
- ↑ Langner, C.; Ratschek, M.; Tsybrovskyy, O.; Schips, L.; Zigeuner, R. (Aug 2003). "P63 immunoreactivity distinguishes upper urinary tract transitional-cell carcinoma and renal-cell carcinoma even in poorly differentiated tumors.". J Histochem Cytochem 51 (8): 1097-9. PMID 12871991.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 600160
- ↑ THvdK. 21 June 2010.
- ↑ Albores-Saavedra J, Chable-Montero F, Hernández-Rodríguez OX, Montante-Montes de Oca D, Angeles-Angeles A (June 2009). "Inverted urothelial papilloma of the urinary bladder with focal papillary pattern: a previously undescribed feature". Ann Diagn Pathol 13 (3): 158–61. doi:10.1016/j.anndiagpath.2009.02.009. PMID 19433293.
- ↑ May M, Brookman-Amissah S, Roigas J, et al. (March 2009). "Prognostic Accuracy of Individual Uropathologists in Noninvasive Urinary Bladder Carcinoma: A Multicentre Study Comparing the 1973 and 2004 World Health Organisation Classifications". Eur. Urol. 57 (5): 850. doi:10.1016/j.eururo.2009.03.052. PMID 19346063.
- ↑ MacLennan GT, Kirkali Z, Cheng L (April 2007). "Histologic grading of noninvasive papillary urothelial neoplasms". Eur. Urol. 51 (4): 889–97; discussion 897–8. doi:10.1016/j.eururo.2006.10.037. PMID 17095142.
- ↑ Jones TD, Cheng L (June 2006). "Papillary urothelial neoplasm of low malignant potential: evolving terminology and concepts". J. Urol. 175 (6): 1995–2003. doi:10.1016/S0022-5347(06)00267-9. PMID 16697785.
- ↑ Cheng, L.; Maclennan, GT.; Lopez-Beltran, A. (Dec 2012). "Histologic grading of urothelial carcinoma: a reappraisal.". Hum Pathol 43 (12): 2097-108. doi:10.1016/j.humpath.2012.01.008. PMID 22542126.
- ↑ Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 170. ISBN 978-0443066771.
- ↑ Watts, KE.; Montironi, R.; Mazzucchelli, R.; van der Kwast, T.; Osunkoya, AO.; Stephenson, AJ.; Hansel, DE. (Aug 2012). "Clinicopathologic characteristics of 23 cases of invasive low-grade papillary urothelial carcinoma.". Urology 80 (2): 361-6. doi:10.1016/j.urology.2012.04.010. PMID 22857755.
- ↑ Miyamoto, H.; Brimo, F.; Schultz, L.; Ye, H.; Miller, JS.; Fajardo, DA.; Lee, TK.; Epstein, JI. et al. (Aug 2010). "Low-grade papillary urothelial carcinoma of the urinary bladder: a clinicopathologic analysis of a post-World Health Organization/International Society of Urological Pathology classification cohort from a single academic center.". Arch Pathol Lab Med 134 (8): 1160-3. doi:10.1043/2009-0403-OA.1. PMID 20670136.
- ↑ Isfoss, BL.; Majak, B.; Busch, C.; Braathen, GJ. (Apr 2011). "Simplification of grading papillary urothelial neoplasia using a reduced set of diagnostic features.". Anal Quant Cytol Histol 33 (2): 68-74. PMID 21980608.
- ↑ 33.0 33.1 Rajcani, J.; Kajo, K.; Adamkov, M.; Moravekova, E.; Lauko, L.; Felcanova, D.; Bencat, M. (2013). "Immunohistochemical characterization of urothelial carcinoma.". Bratisl Lek Listy 114 (8): 431-8. PMID 23944616.
- ↑ Pich, A.; Chiusa, L.; Comino, A.; Navone, R. (1994). "Cell proliferation indices, morphometry and DNA flow cytometry provide objective criteria for distinguishing low and high grade bladder carcinomas.". Virchows Arch 424 (2): 143-8. PMID 7910097.
- ↑ 35.0 35.1 Ehdaie, B.; Theodorescu, D. (Jan 2008). "Molecular markers in transitional cell carcinoma of the bladder: New insights into mechanisms and prognosis.". Indian J Urol 24 (1): 61-7. doi:10.4103/0970-1591.38606. PMC 2684226. PMID 19468362. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2684226/.
- ↑ Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 113. ISBN 978-0443066771.
- ↑ 37.0 37.1 37.2 37.3 Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 120. ISBN 978-0443066771.
- ↑ 38.0 38.1 Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 124. ISBN 978-0443066771.
- ↑ 39.0 39.1 Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 122. ISBN 978-0443066771.
- ↑ 40.0 40.1 Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 127. ISBN 978-0443066771.
- ↑ Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 138. ISBN 978-0443066771.
- ↑ 42.0 42.1 Tanaka, T.; Nitta, Y.; Morimoto, K.; Nishikawa, N.; Nishihara, C.; Tamada, S.; Kawashima, H.; Nakatani, T. (2011). "Hyperbaric oxygen therapy for painful bladder syndrome/interstitial cystitis resistant to conventional treatments: long-term results of a case series in Japan.". BMC Urol 11: 11. doi:10.1186/1471-2490-11-11. PMID 21609485.
- ↑ 43.0 43.1 43.2 French, LM.; Bhambore, N. (May 2011). "Interstitial cystitis/painful bladder syndrome.". Am Fam Physician 83 (10): 1175-81. PMID 21568251.
- ↑ 44.0 44.1 44.2 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1028. ISBN 0-7216-0187-1.
- ↑ 45.0 45.1 Volmar, KE.; Chan, TY.; De Marzo, AM.; Epstein, JI. (Sep 2003). "Florid von Brunn nests mimicking urothelial carcinoma: a morphologic and immunohistochemical comparison to the nested variant of urothelial carcinoma.". Am J Surg Pathol 27 (9): 1243-52. PMID 12960809.
- ↑ 46.0 46.1 46.2 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 304. ISBN 978-0781765275.
- ↑ Wasco, MJ.; Daignault, S.; Bradley, D.; Shah, RB. (Feb 2010). "Nested variant of urothelial carcinoma: a clinicopathologic and immunohistochemical study of 30 pure and mixed cases.". Hum Pathol 41 (2): 163-71. doi:10.1016/j.humpath.2009.07.015. PMID 19800100.
- ↑ Chan, YM.; Ka-Leung Cheng, D.; Nga-Yin Cheung, A.; Yuen-Sheung Ngan, H.; Wong, LC. (Dec 2000). "Female urethral adenocarcinoma arising from urethritis glandularis.". Gynecol Oncol 79 (3): 511-4. doi:10.1006/gyno.2000.5968. PMID 11104631.
- ↑ Yin, G.; Liu, YQ.; Gao, P.; Wang, XH. (Aug 2007). "Male urethritis glandularis: case report.". Chin Med J (Engl) 120 (16): 1460-1. PMID 17825180.
- ↑ Singh, KJ. (Jan 2011). "Mesonephric adenoma in remnant ureteric stump: A rare entity.". Indian J Urol 27 (1): 140-1. doi:10.4103/0970-1591.78414. PMID 21716880.
- ↑ 51.0 51.1 51.2 Gokaslan, ST.; Krueger, JE.; Albores-Saavedra, J. (Jul 2002). "Symptomatic nephrogenic metaplasia of ureter: a morphologic and immunohistochemical study of four cases.". Mod Pathol 15 (7): 765-70. doi:10.1097/01.MP.0000019578.51568.24. PMID 12118115. http://www.nature.com/modpathol/journal/v15/n7/full/3880603a.html. Cite error: Invalid
<ref>tag; name "pmid12118115" defined multiple times with different content - ↑ Mazal, PR.; Schaufler, R.; Altenhuber-Müller, R.; Haitel, A.; Watschinger, B.; Kratzik, C.; Krupitza, G.; Regele, H. et al. (Aug 2002). "Derivation of nephrogenic adenomas from renal tubular cells in kidney-transplant recipients.". N Engl J Med 347 (9): 653-9. doi:10.1056/NEJMoa013413. PMID 12200552.
- ↑ Kunju, LP. (Oct 2010). "Nephrogenic adenoma: report of a case and review of morphologic mimics.". Arch Pathol Lab Med 134 (10): 1455-9. doi:10.1043/2010-0226-CR.1. PMID 20923300.
- ↑ Alexiev, BA.; Levea, CM. (Mar 2012). "Nephrogenic Adenoma of the Urinary Tract: A Review.". Int J Surg Pathol. doi:10.1177/1066896912439095. PMID 22415059.