Difference between revisions of "Gastrointestinal stromal tumour"
Jump to navigation
Jump to search
(→Microscopic: tweak) |
m (mutations) |
||
| (33 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{ Infobox diagnosis | |||
| Name = {{PAGENAME}} | |||
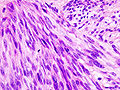
| Image = Gastrointestinal_stromal_tumour_-_very_low_mag.jpg | |||
| Width = | |||
| Caption = Gastrointestinal stromal tumour. [[H&E stain]]. | |||
| Micro = spindle ''or'' epithelioid ''or'' mixed morphology, usu. centred on the muscularis propria | |||
| Subtypes = | |||
| LMDDx = [[schwannoma]], [[leiomyoma]], [[leiomyosarcoma]], [[neurofibroma]], [[desmoid-type fibromatosis]] | |||
| Stains = | |||
| IHC = CD117 +ve, [[DOG1]] +ve, CD34 +ve, S-100 -ve | |||
| EM = | |||
| Molecular = mutation in KIT gene ''or'' PDGFRA gene | |||
| IF = | |||
| Gross = | |||
| Grossing = | |||
| Staging = [[gastrointestinal stromal tumour staging]] | |||
| Site = [[stomach]], [[small intestine]], other sites | |||
| Assdx = | |||
| Syndromes = [[Neurofibromatosis type 1]], [[Carney triad]], [[Carney-Stratakis syndrome]] | |||
| Clinicalhx = | |||
| Signs = | |||
| Symptoms = | |||
| Prevalence = | |||
| Bloodwork = | |||
| Rads = | |||
| Endoscopy = | |||
| Prognosis = good to poor - dependent on size, site & mitotic rate | |||
| Other = | |||
| ClinDDx = | |||
}} | |||
The '''gastrointestinal stromal tumour''', abbreviated '''GIST''', is an uncommon tumour of the [[gastrointestinal tract pathology|gastrointestinal tract]]. | The '''gastrointestinal stromal tumour''', abbreviated '''GIST''', is an uncommon tumour of the [[gastrointestinal tract pathology|gastrointestinal tract]]. | ||
==General== | ==General== | ||
===Definition=== | ===Definition=== | ||
* | *Tumour resulting from a mutation in the KIT gene ''or'' PDGFRA (Platelet-derived growth factor receptor, alpha polypeptide) gene.<ref name=pmid17090188/> | ||
*Cases wild-type for KIT or PDFGRA may harbour defects in the [[succinate dehydrogenase]] complex, NF-1, BRAF, or extremely rarely KRAS. | |||
===Epidemiology=== | ===Epidemiology=== | ||
| Line 11: | Line 42: | ||
*[[Neurofibromatosis|Neurofibromatosis 1]] (von Recklinghausen's disease). | *[[Neurofibromatosis|Neurofibromatosis 1]] (von Recklinghausen's disease). | ||
*[[Carney triad]]. | *[[Carney triad]]. | ||
* | *[[Carney-Stratakis syndrome]] - GISTs and [[paraganglioma]] - due to mutation in the genes for [[succinate dehydrogenase]].<ref name=pmid22997454>{{Cite journal | last1 = Blay | first1 = JY. | last2 = Blomqvist | first2 = C. | last3 = Bonvalot | first3 = S. | last4 = Boukovinas | first4 = I. | last5 = Casali | first5 = PG. | last6 = De Alava | first6 = E. | last7 = Dei Tos | first7 = AP. | last8 = Dirksen | first8 = U. | last9 = Duffaud | first9 = F. | title = Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. | journal = Ann Oncol | volume = 23 Suppl 7 | issue = | pages = vii49-55 | month = Oct | year = 2012 | doi = 10.1093/annonc/mds252 | PMID = 22997454 | url = http://annonc.oxfordjournals.org/content/23/suppl_7/vii49.full }}</ref> | ||
===Treatment=== | ===Treatment=== | ||
| Line 22: | Line 52: | ||
*Large size. | *Large size. | ||
**Often benign if small size. | **Often benign if small size. | ||
*High mitotic rate (for area 5mm | *High mitotic rate (for area 5mm<sup>2</sup>). | ||
*Site - small intestine GISTs worse than stomach GISTs. | *Site - small intestine GISTs worse than stomach GISTs. | ||
Small intestine bad prognosis:<ref name=pmid17090188/> | Small intestine bad prognosis:<ref name=pmid17090188/> | ||
* >5 mitoses/5 mm | * >5 mitoses/5 mm<sup>2</sup> ''or'' size >10 cm. | ||
Stomach bad prognosis:<ref name=pmid17090188/> | Stomach bad prognosis:<ref name=pmid17090188/> | ||
* >5 mitoses/5 mm | * >5 mitoses/5 mm<sup>2</sup> ''and'' size >5 cm. | ||
===Location=== | ===Location=== | ||
| Line 39: | Line 69: | ||
Notes: | Notes: | ||
*Small intestinal GISTs have a worse prognosis than gastric ones.<ref name=pmid17090188/> | *Small intestinal GISTs have a worse prognosis than gastric ones.<ref name=pmid17090188/> | ||
*GISTs almost never metastasize to the [[lymph node]]s | *GISTs almost never metastasize to the [[lymph node]]s (except for SDH-B deficient epithelioid GISTs) | ||
**Most common [[metastasis]] locations: [[liver]], abdominal soft tissue. | **Most common [[metastasis]] locations: [[liver]], abdominal soft tissue. | ||
| Line 47: | Line 77: | ||
** May be epithelioid (round) ~40% of tumours. | ** May be epithelioid (round) ~40% of tumours. | ||
** Mixed epithelioid and spindle cell tumours ~10% tumours. | ** Mixed epithelioid and spindle cell tumours ~10% tumours. | ||
*+/-Cytoplasmic inclusions | *+/-Cytoplasmic inclusions<ref name=pmid7757951>{{cite journal |author=Pasquinelli G, Severi B, Martinelli GN, Santini D, Gelli MC, Tison V |title=Gastro-intestinal stromal tumors: an ultrastructural reinterpretation of the clear cell component |journal=J. Submicrosc. Cytol. Pathol. |volume=27 |issue=2 |pages=251–7 |year=1995 |month=April |pmid=7757951 |doi= |url=}}</ref> - perinuclear.<ref>{{Cite journal | last1 = Boşoteanu | first1 = M. | last2 = Boşoteanu | first2 = C. | last3 = Deacu | first3 = M. | last4 = Aşchie | first4 = M. | title = Differential diagnosis of a gastric stromal tumor: case report and literature review. | journal = Rom J Morphol Embryol | volume = 52 | issue = 4 | pages = 1361-8 | month = | year = 2011 | doi = | PMID = 22203947 }}</ref> | ||
*Classically splits the layers of the ''muscularis propria'' - as this is where the ''interstitial cells of Cajal'' are located.<ref name=pmid16402273>{{cite journal |author=Agaimy A, Wünsch PH |title=Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours |journal=Langenbecks Arch Surg |volume=391 |issue=4 |pages=322–9 |year=2006 |month=August |pmid=16402273 |doi=10.1007/s00423-005-0005-5 |url=}}</ref> | *Classically splits the layers of the ''muscularis propria'' - as this is where the ''interstitial cells of Cajal'' are located.<ref name=pmid16402273>{{cite journal |author=Agaimy A, Wünsch PH |title=Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours |journal=Langenbecks Arch Surg |volume=391 |issue=4 |pages=322–9 |year=2006 |month=August |pmid=16402273 |doi=10.1007/s00423-005-0005-5 |url=}}</ref> | ||
*+/-Skenoid fibres - extracellular collagen bundles<ref name=pmid15798063/> ~ 2-5 x 60 micrometers - uncommon finding. | *+/-Skenoid fibres - extracellular collagen bundles<ref name=pmid15798063/> ~ 2-5 x 60 micrometers - uncommon finding. | ||
**Not seen in gastric GISTs.<ref name=pmid12692202/> | **Not seen in gastric GISTs.<ref name=pmid12692202/> | ||
| Line 61: | Line 91: | ||
***GFAP uniformly neg. in GISTs.<ref name=pmid17090188/> | ***GFAP uniformly neg. in GISTs.<ref name=pmid17090188/> | ||
*[[Desmoid-type fibromatosis]]. | *[[Desmoid-type fibromatosis]]. | ||
*[[Epstein-Barr virus-associated smooth muscle tumour]] - very uncommon, in immunoincompetent individuals.<ref name=pmid16330945>{{Cite journal | last1 = Deyrup | first1 = AT. | last2 = Lee | first2 = VK. | last3 = Hill | first3 = CE. | last4 = Cheuk | first4 = W. | last5 = Toh | first5 = HC. | last6 = Kesavan | first6 = S. | last7 = Chan | first7 = EW. | last8 = Weiss | first8 = SW. | title = Epstein-Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients. | journal = Am J Surg Pathol | volume = 30 | issue = 1 | pages = 75-82 | month = Jan | year = 2006 | doi = | PMID = 16330945 }}</ref> | |||
Images | ===Images=== | ||
*www: | *www: | ||
**[http://radiographics.rsna.org/content/25/2/455/F67.expansion.html GIST (radiographics.rsna.org)].<ref name=pmid15798063>{{Cite journal | last1 = Levy | first1 = AD. | last2 = Patel | first2 = N. | last3 = Dow | first3 = N. | last4 = Abbott | first4 = RM. | last5 = Miettinen | first5 = M. | last6 = Sobin | first6 = LH. | title = From the archives of the AFIP: abdominal neoplasms in patients with neurofibromatosis type 1: radiologic-pathologic correlation. | journal = Radiographics | volume = 25 | issue = 2 | pages = 455-80 | month = | year = | doi = 10.1148/rg.252045176 | PMID = 15798063 |URL = http://radiographics.rsnajnls.org/cgi/pmidlookup?view=long&pmid=15798063}}</ref> | **[http://radiographics.rsna.org/content/25/2/455/F67.expansion.html GIST (radiographics.rsna.org)].<ref name=pmid15798063>{{Cite journal | last1 = Levy | first1 = AD. | last2 = Patel | first2 = N. | last3 = Dow | first3 = N. | last4 = Abbott | first4 = RM. | last5 = Miettinen | first5 = M. | last6 = Sobin | first6 = LH. | title = From the archives of the AFIP: abdominal neoplasms in patients with neurofibromatosis type 1: radiologic-pathologic correlation. | journal = Radiographics | volume = 25 | issue = 2 | pages = 455-80 | month = | year = | doi = 10.1148/rg.252045176 | PMID = 15798063 |URL = http://radiographics.rsnajnls.org/cgi/pmidlookup?view=long&pmid=15798063}}</ref> | ||
**[http://radiographics.rsna.org/content/25/2/455/F68.expansion.html GIST with skenoid fibres (radiographics.rsna.org)].<ref name=pmid15798063/> | **[http://radiographics.rsna.org/content/25/2/455/F68.expansion.html GIST with skenoid fibres (radiographics.rsna.org)].<ref name=pmid15798063/> | ||
**[http://www.nature.com/modpathol/journal/v16/n4/fig_tab/3880774f6.html GIST with skenoid fibres (nature.com)].<ref name=pmid12692202>{{Cite journal | last1 = Greenson | first1 = JK. | title = Gastrointestinal stromal tumors and other mesenchymal lesions of the gut. | journal = Mod Pathol | volume = 16 | issue = 4 | pages = 366-75 | month = Apr | year = 2003 | doi = 10.1097/01.MP.0000062860.60390.C7 | PMID = 12692202 | URL = http://www.nature.com/modpathol/journal/v16/n4/full/3880774a.html}}</ref> | **[http://www.nature.com/modpathol/journal/v16/n4/fig_tab/3880774f6.html GIST with skenoid fibres (nature.com)].<ref name=pmid12692202>{{Cite journal | last1 = Greenson | first1 = JK. | title = Gastrointestinal stromal tumors and other mesenchymal lesions of the gut. | journal = Mod Pathol | volume = 16 | issue = 4 | pages = 366-75 | month = Apr | year = 2003 | doi = 10.1097/01.MP.0000062860.60390.C7 | PMID = 12692202 | URL = http://www.nature.com/modpathol/journal/v16/n4/full/3880774a.html}}</ref> | ||
<gallery> | |||
Image:Gastric_GIST_%282%29.jpg | GIST - low mag. (WC/KGH) | |||
Image:Gastric_GIST_%281%29.jpg | GIST - high mag. (WC/KGH) | |||
</gallery> | |||
<gallery> | |||
Image:Gastrointestinal_stromal_tumour_-_very_low_mag.jpg | Intestinal spindle cell GIST - very low mag. (WC/Nephron) | |||
Image:Gastrointestinal_stromal_tumour_-_low_mag.jpg | Intestinal spindle cell GIST - low mag. (WC/Nephron) | |||
Image:Gastrointestinal_stromal_tumour_-_intermed_mag.jpg | Intestinal spindle cell GIST - intermed. mag. (WC/Nephron) | |||
Image:Gastrointestinal_stromal_tumour_-_high_mag.jpg | Intestinal spindle cell GIST - high mag. (WC/Nephron) | |||
Image:Gastrointestinal_stromal_tumour_-_very_high_mag.jpg | Intestinal spindle cell GIST - very high mag. (WC/Nephron) | |||
Image:Gastrointestinal_stromal_tumour_-_superf_-_intermed_mag.jpg | Epithelioid GIST - intermed. mag. (WC/Nephron) | |||
</gallery> | |||
==IHC== | ==IHC== | ||
*CD117 +ve in 95%.<ref name=pmid17090188/> | *CD117 +ve in 95%.<ref name=pmid17090188/> | ||
**[[Mast cell]]s are the internal positive control. | **[[Mast cell]]s are the internal positive control. | ||
*[[DOG1]] +ve.<ref name=pmid19011564>{{Cite journal | last1 = Liegl | first1 = B. | last2 = Hornick | first2 = JL. | last3 = Corless | first3 = CL. | last4 = Fletcher | first4 = CD. | title = Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. | journal = Am J Surg Pathol | volume = 33 | issue = 3 | pages = 437-46 | month = Mar | year = 2009 | doi = 10.1097/PAS.0b013e318186b158 | PMID = 19011564 }}</ref> | |||
Others: | |||
*CD34 +ve in 70%.<ref name=pmid17090188/> | |||
*Desmin +ve in 5%.<ref name=pmid17090188/> | *Desmin +ve in 5%.<ref name=pmid17090188/> | ||
* | *WT1 +ve -- cytoplasmic (28/28 cases<ref name=pmid18528287>{{Cite journal | last1 = Bing | first1 = Z. | last2 = Pasha | first2 = TL. | last3 = Acs | first3 = G. | last4 = Zhang | first4 = PJ. | title = Cytoplasmic overexpression of WT-1 in gastrointestinal stromal tumor and other soft tissue tumors. | journal = Appl Immunohistochem Mol Morphol | volume = 16 | issue = 4 | pages = 316-21 | month = Jul | year = 2008 | doi = 10.1097/PAI.0b013e31815c2e02 | PMID = 18528287 }}</ref>). | ||
===IHC work-up panel=== | |||
=== | |||
*S-100 (neural tumours, rarely +ve in GISTs<ref name=pmid17090188/>). | *S-100 (neural tumours, rarely +ve in GISTs<ref name=pmid17090188/>). | ||
*CD34, CD117 (GIST). | *CD34, CD117 (GIST). | ||
| Line 93: | Line 130: | ||
*Sequence Kit gene, PDGFRA gene. | *Sequence Kit gene, PDGFRA gene. | ||
**Kit gene sequencing is being done more frequently as of late-- if a mutation is found it suggest the drug ''[[imatinib]]'' will be effective. | **Kit gene sequencing is being done more frequently as of late-- if a mutation is found it suggest the drug ''[[imatinib]]'' will be effective. | ||
**Exon 11 mutation associated with malignant behaviour.<ref name=pmid9916918>{{Cite journal | last1 = Lasota | first1 = J. | last2 = Jasinski | first2 = M. | last3 = Sarlomo-Rikala | first3 = M. | last4 = Miettinen | first4 = M. | title = Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. | journal = Am J Pathol | volume = 154 | issue = 1 | pages = 53-60 | month = Jan | year = 1999 | doi = 10.1016/S0002-9440(10)65250-9 | PMID = 9916918 }}</ref> | |||
**Secondary mutations of c-kit lead to imatinib resistance,<ref name=pmid26779618>{{Cite journal | last1 = Wada | first1 = N. | last2 = Kurokawa | first2 = Y. | last3 = Takahashi | first3 = T. | last4 = Hamakawa | first4 = T. | last5 = Hirota | first5 = S. | last6 = Naka | first6 = T. | last7 = Miyazaki | first7 = Y. | last8 = Makino | first8 = T. | last9 = Yamasaki | first9 = M. | title = Detecting Secondary C-KIT Mutations in the Peripheral Blood of Patients with Imatinib-Resistant Gastrointestinal Stromal Tumor. | journal = Oncology | volume = 90 | issue = 2 | pages = 112-7 | month = | year = 2016 | doi = 10.1159/000442948 | PMID = 26779618 }}</ref> and resistance to other similar inhibitors. | |||
==Gastrointestinal stromal tumour staging== | |||
{{Main|Gastrointestinal stromal tumour staging}} | |||
GIST has its own staging. | |||
==Sign out== | |||
<pre> | |||
STOMACH (MASS), LESSER CURVE, WEDGE RESECTION: | |||
- GASTROINTESTINAL STROMAL TUMOUR (GIST). | |||
-- MARGINS NEGATIVE FOR GIST. | |||
COMMENT: | |||
The tumour stains as follows: | |||
POSITIVE: CD117, CD34. | |||
NEGATIVE: Desmin, S-100. | |||
</pre> | |||
<pre> | |||
SMALL BOWEL (ILEUM), RESECTION: | |||
- GASTROINTESTINAL STROMAL TUMOUR (GIST), LOW-GRADE, NO RISK OF | |||
PROGRESSIVE DISEASE. | |||
-- MARGINS NEGATIVE FOR GIST. | |||
-- PLEASE SEE TUMOUR SUMMARY. | |||
- THREE BENIGN LYMPH NODES. | |||
COMMENT: | |||
The tumour stains as follows: | |||
POSITIVE: CD117, CD34. | |||
NEGATIVE: Desmin, S-100. | |||
PROLIFERATION (Ki-67): <1%. | |||
</pre> | |||
====Incidental GIST==== | |||
<pre> | |||
Partial Stomach, Sleeve Gastrectomy: | |||
- Stomach wall with incidental GASTROINTESTINAL STROMAL TUMOUR (GIST), 2 mm in maximal dimension. | |||
-- Margin clear. | |||
- Gastric mucosa within normal limits. | |||
Comment: | |||
The tumour stains as follows: | |||
POSITIVE: DOG1, CD117, CD34. | |||
NEGATIVE: desmin, S-100. | |||
PROLIFERATION (Ki-67): <2%. | |||
</pre> | |||
===Staging=== | |||
*The stage is primarily determined by the tumour size and mitotic grade. | |||
**In the stomach, the mitotic grade determines whether a given tumour is Stage I or Stage III.<ref>{{Cite journal | last1 = Coccolini | first1 = F. | last2 = Catena | first2 = F. | last3 = Ansaloni | first3 = L. | last4 = Pinna | first4 = AD. | title = Gastrointestinal stromal tumor and mitosis, pay attention. | journal = World J Gastroenterol | volume = 18 | issue = 6 | pages = 587-8 | month = Feb | year = 2012 | doi = 10.3748/wjg.v18.i6.587 | PMID = 22363128 }}</ref> | |||
===Micro=== | |||
The sections show a spindle cell lesion that is well-circumscribed and without significant | |||
nuclear pleomorphism. No lymphocytic cuff is surrounding the lesion. The lesion is focally | |||
seen at the inked soft tissue margin. Three mitoses are seen in 5 mm*mm. | |||
==See also== | ==See also== | ||
Latest revision as of 17:28, 15 November 2020
| Gastrointestinal stromal tumour | |
|---|---|
| Diagnosis in short | |
 Gastrointestinal stromal tumour. H&E stain. | |
|
| |
| LM | spindle or epithelioid or mixed morphology, usu. centred on the muscularis propria |
| LM DDx | schwannoma, leiomyoma, leiomyosarcoma, neurofibroma, desmoid-type fibromatosis |
| IHC | CD117 +ve, DOG1 +ve, CD34 +ve, S-100 -ve |
| Molecular | mutation in KIT gene or PDGFRA gene |
| Staging | gastrointestinal stromal tumour staging |
| Site | stomach, small intestine, other sites |
|
| |
| Syndromes | Neurofibromatosis type 1, Carney triad, Carney-Stratakis syndrome |
|
| |
| Prognosis | good to poor - dependent on size, site & mitotic rate |
The gastrointestinal stromal tumour, abbreviated GIST, is an uncommon tumour of the gastrointestinal tract.
General
Definition
- Tumour resulting from a mutation in the KIT gene or PDGFRA (Platelet-derived growth factor receptor, alpha polypeptide) gene.[1]
- Cases wild-type for KIT or PDFGRA may harbour defects in the succinate dehydrogenase complex, NF-1, BRAF, or extremely rarely KRAS.
Epidemiology
- Arise from Interstitial cells of Cajal.[1]
May be familial/syndromic:[2]
- Neurofibromatosis 1 (von Recklinghausen's disease).
- Carney triad.
- Carney-Stratakis syndrome - GISTs and paraganglioma - due to mutation in the genes for succinate dehydrogenase.[3]
Treatment
- Imatinib (Gleevec) - drug was developed for chronic myelogenous leukemia.
Factors predictive of malignant behaviour
Features suggesting a bad prognosis:[1]
- Large size.
- Often benign if small size.
- High mitotic rate (for area 5mm2).
- Site - small intestine GISTs worse than stomach GISTs.
Small intestine bad prognosis:[1]
- >5 mitoses/5 mm2 or size >10 cm.
Stomach bad prognosis:[1]
- >5 mitoses/5 mm2 and size >5 cm.
Location
Most common locations in order:[1]
- 60% in stomach.
- 35% in small intestine.
- 5% elsewhere.
Notes:
- Small intestinal GISTs have a worse prognosis than gastric ones.[1]
- GISTs almost never metastasize to the lymph nodes (except for SDH-B deficient epithelioid GISTs)
- Most common metastasis locations: liver, abdominal soft tissue.
Microscopic
Features:
- Classically, spindle cell morphology ~ 50% of tumours.[4]
- May be epithelioid (round) ~40% of tumours.
- Mixed epithelioid and spindle cell tumours ~10% tumours.
- +/-Cytoplasmic inclusions[5] - perinuclear.[6]
- Classically splits the layers of the muscularis propria - as this is where the interstitial cells of Cajal are located.[7]
- +/-Skenoid fibres - extracellular collagen bundles[8] ~ 2-5 x 60 micrometers - uncommon finding.
- Not seen in gastric GISTs.[9]
- High specificity for GIST.
DDx
- Leiomyosarcoma.
- Leiomyoma - esp. in the esophagus.
- Neural tumours.
- Neurofibroma.
- Schwannoma (GFAP +ve).
- GFAP uniformly neg. in GISTs.[1]
- Desmoid-type fibromatosis.
- Epstein-Barr virus-associated smooth muscle tumour - very uncommon, in immunoincompetent individuals.[10]
Images
- www:
IHC
- CD117 +ve in 95%.[1]
- Mast cells are the internal positive control.
- DOG1 +ve.[11]
Others:
IHC work-up panel
- S-100 (neural tumours, rarely +ve in GISTs[1]).
- CD34, CD117 (GIST).
- Desmin (muscle tumours).
Molecular tests
- Sequence Kit gene, PDGFRA gene.
Gastrointestinal stromal tumour staging
Main article: Gastrointestinal stromal tumour staging
GIST has its own staging.
Sign out
STOMACH (MASS), LESSER CURVE, WEDGE RESECTION: - GASTROINTESTINAL STROMAL TUMOUR (GIST). -- MARGINS NEGATIVE FOR GIST. COMMENT: The tumour stains as follows: POSITIVE: CD117, CD34. NEGATIVE: Desmin, S-100.
SMALL BOWEL (ILEUM), RESECTION: - GASTROINTESTINAL STROMAL TUMOUR (GIST), LOW-GRADE, NO RISK OF PROGRESSIVE DISEASE. -- MARGINS NEGATIVE FOR GIST. -- PLEASE SEE TUMOUR SUMMARY. - THREE BENIGN LYMPH NODES. COMMENT: The tumour stains as follows: POSITIVE: CD117, CD34. NEGATIVE: Desmin, S-100. PROLIFERATION (Ki-67): <1%.
Incidental GIST
Partial Stomach, Sleeve Gastrectomy: - Stomach wall with incidental GASTROINTESTINAL STROMAL TUMOUR (GIST), 2 mm in maximal dimension. -- Margin clear. - Gastric mucosa within normal limits. Comment: The tumour stains as follows: POSITIVE: DOG1, CD117, CD34. NEGATIVE: desmin, S-100. PROLIFERATION (Ki-67): <2%.
Staging
- The stage is primarily determined by the tumour size and mitotic grade.
- In the stomach, the mitotic grade determines whether a given tumour is Stage I or Stage III.[15]
Micro
The sections show a spindle cell lesion that is well-circumscribed and without significant nuclear pleomorphism. No lymphocytic cuff is surrounding the lesion. The lesion is focally seen at the inked soft tissue margin. Three mitoses are seen in 5 mm*mm.
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Miettinen M, Lasota J (October 2006). "Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis". Arch. Pathol. Lab. Med. 130 (10): 1466–78. PMID 17090188. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=130&page=1466.
- ↑ Agaimy A, Hartmann A (October 2010). "[Hereditary and non-hereditary syndromic gastointestinal stromal tumours]" (in German). Pathologe 31 (6): 430–7. doi:10.1007/s00292-010-1354-6. PMID 20848108.
- ↑ Blay, JY.; Blomqvist, C.; Bonvalot, S.; Boukovinas, I.; Casali, PG.; De Alava, E.; Dei Tos, AP.; Dirksen, U. et al. (Oct 2012). "Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.". Ann Oncol 23 Suppl 7: vii49-55. doi:10.1093/annonc/mds252. PMID 22997454. http://annonc.oxfordjournals.org/content/23/suppl_7/vii49.full.
- ↑ Miettinen, M.; Sobin, LH.; Lasota, J. (Jan 2005). "Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up.". Am J Surg Pathol 29 (1): 52-68. PMID 15613856.
- ↑ Pasquinelli G, Severi B, Martinelli GN, Santini D, Gelli MC, Tison V (April 1995). "Gastro-intestinal stromal tumors: an ultrastructural reinterpretation of the clear cell component". J. Submicrosc. Cytol. Pathol. 27 (2): 251–7. PMID 7757951.
- ↑ Boşoteanu, M.; Boşoteanu, C.; Deacu, M.; Aşchie, M. (2011). "Differential diagnosis of a gastric stromal tumor: case report and literature review.". Rom J Morphol Embryol 52 (4): 1361-8. PMID 22203947.
- ↑ Agaimy A, Wünsch PH (August 2006). "Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours". Langenbecks Arch Surg 391 (4): 322–9. doi:10.1007/s00423-005-0005-5. PMID 16402273.
- ↑ 8.0 8.1 8.2 Levy, AD.; Patel, N.; Dow, N.; Abbott, RM.; Miettinen, M.; Sobin, LH.. "From the archives of the AFIP: abdominal neoplasms in patients with neurofibromatosis type 1: radiologic-pathologic correlation.". Radiographics 25 (2): 455-80. doi:10.1148/rg.252045176. PMID 15798063.
- ↑ 9.0 9.1 Greenson, JK. (Apr 2003). "Gastrointestinal stromal tumors and other mesenchymal lesions of the gut.". Mod Pathol 16 (4): 366-75. doi:10.1097/01.MP.0000062860.60390.C7. PMID 12692202.
- ↑ Deyrup, AT.; Lee, VK.; Hill, CE.; Cheuk, W.; Toh, HC.; Kesavan, S.; Chan, EW.; Weiss, SW. (Jan 2006). "Epstein-Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients.". Am J Surg Pathol 30 (1): 75-82. PMID 16330945.
- ↑ Liegl, B.; Hornick, JL.; Corless, CL.; Fletcher, CD. (Mar 2009). "Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes.". Am J Surg Pathol 33 (3): 437-46. doi:10.1097/PAS.0b013e318186b158. PMID 19011564.
- ↑ Bing, Z.; Pasha, TL.; Acs, G.; Zhang, PJ. (Jul 2008). "Cytoplasmic overexpression of WT-1 in gastrointestinal stromal tumor and other soft tissue tumors.". Appl Immunohistochem Mol Morphol 16 (4): 316-21. doi:10.1097/PAI.0b013e31815c2e02. PMID 18528287.
- ↑ Lasota, J.; Jasinski, M.; Sarlomo-Rikala, M.; Miettinen, M. (Jan 1999). "Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas.". Am J Pathol 154 (1): 53-60. doi:10.1016/S0002-9440(10)65250-9. PMID 9916918.
- ↑ Wada, N.; Kurokawa, Y.; Takahashi, T.; Hamakawa, T.; Hirota, S.; Naka, T.; Miyazaki, Y.; Makino, T. et al. (2016). "Detecting Secondary C-KIT Mutations in the Peripheral Blood of Patients with Imatinib-Resistant Gastrointestinal Stromal Tumor.". Oncology 90 (2): 112-7. doi:10.1159/000442948. PMID 26779618.
- ↑ Coccolini, F.; Catena, F.; Ansaloni, L.; Pinna, AD. (Feb 2012). "Gastrointestinal stromal tumor and mitosis, pay attention.". World J Gastroenterol 18 (6): 587-8. doi:10.3748/wjg.v18.i6.587. PMID 22363128.