Difference between revisions of "Gastrointestinal stromal tumour"
Jump to navigation
Jump to search
| Line 76: | Line 76: | ||
** May be epithelioid (round) ~40% of tumours. | ** May be epithelioid (round) ~40% of tumours. | ||
** Mixed epithelioid and spindle cell tumours ~10% tumours. | ** Mixed epithelioid and spindle cell tumours ~10% tumours. | ||
*+/-Cytoplasmic inclusions<ref name=pmid7757951>{{cite journal |author=Pasquinelli G, Severi B, Martinelli GN, Santini D, Gelli MC, Tison V |title=Gastro-intestinal stromal tumors: an ultrastructural reinterpretation of the clear cell component |journal=J. Submicrosc. Cytol. Pathol. |volume=27 |issue=2 |pages=251–7 |year=1995 |month=April |pmid=7757951 |doi= |url=}}</ref> | *+/-Cytoplasmic inclusions<ref name=pmid7757951>{{cite journal |author=Pasquinelli G, Severi B, Martinelli GN, Santini D, Gelli MC, Tison V |title=Gastro-intestinal stromal tumors: an ultrastructural reinterpretation of the clear cell component |journal=J. Submicrosc. Cytol. Pathol. |volume=27 |issue=2 |pages=251–7 |year=1995 |month=April |pmid=7757951 |doi= |url=}}</ref> - perinuclear.<ref>{{Cite journal | last1 = Boşoteanu | first1 = M. | last2 = Boşoteanu | first2 = C. | last3 = Deacu | first3 = M. | last4 = Aşchie | first4 = M. | title = Differential diagnosis of a gastric stromal tumor: case report and literature review. | journal = Rom J Morphol Embryol | volume = 52 | issue = 4 | pages = 1361-8 | month = | year = 2011 | doi = | PMID = 22203947 }}</ref> | ||
*Classically splits the layers of the ''muscularis propria'' - as this is where the ''interstitial cells of Cajal'' are located.<ref name=pmid16402273>{{cite journal |author=Agaimy A, Wünsch PH |title=Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours |journal=Langenbecks Arch Surg |volume=391 |issue=4 |pages=322–9 |year=2006 |month=August |pmid=16402273 |doi=10.1007/s00423-005-0005-5 |url= | *Classically splits the layers of the ''muscularis propria'' - as this is where the ''interstitial cells of Cajal'' are located.<ref name=pmid16402273>{{cite journal |author=Agaimy A, Wünsch PH |title=Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours |journal=Langenbecks Arch Surg |volume=391 |issue=4 |pages=322–9 |year=2006 |month=August |pmid=16402273 |doi=10.1007/s00423-005-0005-5 |url=}}</ref> | ||
*+/-Skenoid fibres - extracellular collagen bundles<ref name=pmid15798063/> ~ 2-5 x 60 micrometers - uncommon finding. | *+/-Skenoid fibres - extracellular collagen bundles<ref name=pmid15798063/> ~ 2-5 x 60 micrometers - uncommon finding. | ||
**Not seen in gastric GISTs.<ref name=pmid12692202/> | **Not seen in gastric GISTs.<ref name=pmid12692202/> | ||
Revision as of 16:25, 10 July 2016
| Gastrointestinal stromal tumour | |
|---|---|
| Diagnosis in short | |
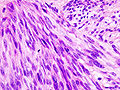
 Gastrointestinal stromal tumour. H&E stain. | |
|
| |
| LM | spindle or epithelioid or mixed morphology, usu. centred on the muscularis propria |
| LM DDx | schwannoma, leiomyoma, leiomyosarcoma, neurofibroma, desmoid-type fibromatosis |
| IHC | CD117 +ve, DOG1 +ve, CD34 +ve, S-100 -ve |
| Molecular | mutation in KIT gene or PDGFRA gene |
| Staging | gastrointestinal stromal tumour staging |
| Site | stomach, small intestine, other sites |
|
| |
| Syndromes | Neurofibromatosis type 1, Carney triad, Carney-Stratakis syndrome |
|
| |
| Prognosis | good to poor - dependent on size, site & mitotic rate |
The gastrointestinal stromal tumour, abbreviated GIST, is an uncommon tumour of the gastrointestinal tract.
General
Definition
- Tumour resulting from a mutation in the KIT gene or PDGFRA (Platelet-derived growth factor receptor, alpha polypeptide) gene.[1]
Epidemiology
- Arise from Interstitial cells of Cajal.[1]
May be familial/syndromic:[2]
- Neurofibromatosis 1 (von Recklinghausen's disease).
- Carney triad.
- Carney-Stratakis syndrome - GISTs and paraganglioma - due to mutation in the genes for succinate dehydrogenase.[3]
Treatment
- Imatinib (Gleevec) - drug was developed for chronic myelogenous leukemia.
Factors predictive of malignant behaviour
Features suggesting a bad prognosis:[1]
- Large size.
- Often benign if small size.
- High mitotic rate (for area 5mm2).
- Site - small intestine GISTs worse than stomach GISTs.
Small intestine bad prognosis:[1]
- >5 mitoses/5 mm2 or size >10 cm.
Stomach bad prognosis:[1]
- >5 mitoses/5 mm2 and size >5 cm.
Location
Most common locations in order:[1]
- 60% in stomach.
- 35% in small intestine.
- 5% elsewhere.
Notes:
- Small intestinal GISTs have a worse prognosis than gastric ones.[1]
- GISTs almost never metastasize to the lymph nodes.
- Most common metastasis locations: liver, abdominal soft tissue.
Microscopic
Features:
- Classically, spindle cell morphology ~ 50% of tumours.[4]
- May be epithelioid (round) ~40% of tumours.
- Mixed epithelioid and spindle cell tumours ~10% tumours.
- +/-Cytoplasmic inclusions[5] - perinuclear.[6]
- Classically splits the layers of the muscularis propria - as this is where the interstitial cells of Cajal are located.[7]
- +/-Skenoid fibres - extracellular collagen bundles[8] ~ 2-5 x 60 micrometers - uncommon finding.
- Not seen in gastric GISTs.[9]
- High specificity for GIST.
DDx
- Leiomyosarcoma.
- Leiomyoma - esp. in the esophagus.
- Neural tumours.
- Neurofibroma.
- Schwannoma (GFAP +ve).
- GFAP uniformly neg. in GISTs.[1]
- Desmoid-type fibromatosis.
- Epstein-Barr virus-associated smooth muscle tumour - very uncommon, in immunoincompetent individuals.[10]
Images
- www:
IHC
- CD117 +ve in 95%.[1]
- Mast cells are the internal positive control.
- DOG1 +ve.[11]
Others:
IHC work-up panel
- S-100 (neural tumours, rarely +ve in GISTs[1]).
- CD34, CD117 (GIST).
- Desmin (muscle tumours).
Molecular tests
- Sequence Kit gene, PDGFRA gene.
Gastrointestinal stromal tumour staging
Main article: Gastrointestinal stromal tumour staging
GIST has its own staging.
Sign out
STOMACH (MASS), LESSER CURVE, WEDGE RESECTION: - GASTROINTESTINAL STROMAL TUMOUR (GIST). -- MARGINS NEGATIVE FOR GIST. COMMENT: The tumour stains as follows: POSITIVE: CD117, CD34. NEGATIVE: Desmin, S-100.
SMALL BOWEL (ILEUM), RESECTION: - GASTROINTESTINAL STROMAL TUMOUR (GIST), LOW-GRADE, NO RISK OF PROGRESSIVE DISEASE. -- MARGINS NEGATIVE FOR GIST. -- PLEASE SEE TUMOUR SUMMARY. - THREE BENIGN LYMPH NODES. COMMENT: The tumour stains as follows: POSITIVE: CD117, CD34. NEGATIVE: Desmin, S-100. PROLIFERATION (Ki-67): <1%.
Staging
- The stage is primarily determined by the tumour size and mitotic grade.
- In the stomach, the mitotic grade determines whether a given tumour is Stage I or Stage III.[15]
Micro
The sections show a spindle cell lesion that is well-circumscribed and without significant nuclear pleomorphism. No lymphocytic cuff is surrounding the lesion. The lesion is focally seen at the inked soft tissue margin. Three mitoses are seen in 5 mm*mm.
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Miettinen M, Lasota J (October 2006). "Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis". Arch. Pathol. Lab. Med. 130 (10): 1466–78. PMID 17090188. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=130&page=1466.
- ↑ Agaimy A, Hartmann A (October 2010). "[Hereditary and non-hereditary syndromic gastointestinal stromal tumours]" (in German). Pathologe 31 (6): 430–7. doi:10.1007/s00292-010-1354-6. PMID 20848108.
- ↑ Blay, JY.; Blomqvist, C.; Bonvalot, S.; Boukovinas, I.; Casali, PG.; De Alava, E.; Dei Tos, AP.; Dirksen, U. et al. (Oct 2012). "Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.". Ann Oncol 23 Suppl 7: vii49-55. doi:10.1093/annonc/mds252. PMID 22997454. http://annonc.oxfordjournals.org/content/23/suppl_7/vii49.full.
- ↑ Miettinen, M.; Sobin, LH.; Lasota, J. (Jan 2005). "Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up.". Am J Surg Pathol 29 (1): 52-68. PMID 15613856.
- ↑ Pasquinelli G, Severi B, Martinelli GN, Santini D, Gelli MC, Tison V (April 1995). "Gastro-intestinal stromal tumors: an ultrastructural reinterpretation of the clear cell component". J. Submicrosc. Cytol. Pathol. 27 (2): 251–7. PMID 7757951.
- ↑ Boşoteanu, M.; Boşoteanu, C.; Deacu, M.; Aşchie, M. (2011). "Differential diagnosis of a gastric stromal tumor: case report and literature review.". Rom J Morphol Embryol 52 (4): 1361-8. PMID 22203947.
- ↑ Agaimy A, Wünsch PH (August 2006). "Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours". Langenbecks Arch Surg 391 (4): 322–9. doi:10.1007/s00423-005-0005-5. PMID 16402273.
- ↑ 8.0 8.1 8.2 Levy, AD.; Patel, N.; Dow, N.; Abbott, RM.; Miettinen, M.; Sobin, LH.. "From the archives of the AFIP: abdominal neoplasms in patients with neurofibromatosis type 1: radiologic-pathologic correlation.". Radiographics 25 (2): 455-80. doi:10.1148/rg.252045176. PMID 15798063.
- ↑ 9.0 9.1 Greenson, JK. (Apr 2003). "Gastrointestinal stromal tumors and other mesenchymal lesions of the gut.". Mod Pathol 16 (4): 366-75. doi:10.1097/01.MP.0000062860.60390.C7. PMID 12692202.
- ↑ Deyrup, AT.; Lee, VK.; Hill, CE.; Cheuk, W.; Toh, HC.; Kesavan, S.; Chan, EW.; Weiss, SW. (Jan 2006). "Epstein-Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients.". Am J Surg Pathol 30 (1): 75-82. PMID 16330945.
- ↑ Liegl, B.; Hornick, JL.; Corless, CL.; Fletcher, CD. (Mar 2009). "Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes.". Am J Surg Pathol 33 (3): 437-46. doi:10.1097/PAS.0b013e318186b158. PMID 19011564.
- ↑ Bing, Z.; Pasha, TL.; Acs, G.; Zhang, PJ. (Jul 2008). "Cytoplasmic overexpression of WT-1 in gastrointestinal stromal tumor and other soft tissue tumors.". Appl Immunohistochem Mol Morphol 16 (4): 316-21. doi:10.1097/PAI.0b013e31815c2e02. PMID 18528287.
- ↑ Lasota, J.; Jasinski, M.; Sarlomo-Rikala, M.; Miettinen, M. (Jan 1999). "Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas.". Am J Pathol 154 (1): 53-60. doi:10.1016/S0002-9440(10)65250-9. PMID 9916918.
- ↑ Wada, N.; Kurokawa, Y.; Takahashi, T.; Hamakawa, T.; Hirota, S.; Naka, T.; Miyazaki, Y.; Makino, T. et al. (2016). "Detecting Secondary C-KIT Mutations in the Peripheral Blood of Patients with Imatinib-Resistant Gastrointestinal Stromal Tumor.". Oncology 90 (2): 112-7. doi:10.1159/000442948. PMID 26779618.
- ↑ Coccolini, F.; Catena, F.; Ansaloni, L.; Pinna, AD. (Feb 2012). "Gastrointestinal stromal tumor and mitosis, pay attention.". World J Gastroenterol 18 (6): 587-8. doi:10.3748/wjg.v18.i6.587. PMID 22363128.