Difference between revisions of "Primitive neuroectodermal tumour"
Jump to navigation
Jump to search
Jensflorian (talk | contribs) (update) |
Jensflorian (talk | contribs) (→CNS neuroblastoma: molecular cluster) |
||
| Line 63: | Line 63: | ||
===CNS neuroblastoma=== | ===CNS neuroblastoma=== | ||
* | * Since WHO 2016 CNS classification this is now a subgroup of [[Other CNS embryonal tumours]]. | ||
* Many CNS neuroblastoma / CNS ganglioneuroblastoma cluster molecularly into a group designated as CNS NB-FOXR2.<ref>{{Cite journal | last1 = Sturm | first1 = D. | last2 = Orr | first2 = BA. | last3 = Toprak | first3 = UH. | last4 = Hovestadt | first4 = V. | last5 = Jones | first5 = DTW. | last6 = Capper | first6 = D. | last7 = Sill | first7 = M. | last8 = Buchhalter | first8 = I. | last9 = Northcott | first9 = PA. | title = New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. | journal = Cell | volume = 164 | issue = 5 | pages = 1060-1072 | month = Feb | year = 2016 | doi = 10.1016/j.cell.2016.01.015 | PMID = 26919435 }}</ref> | |||
*Usu Olig-2 +ve. | |||
*Synaptophysin +ve. | |||
===CNS ganglioneuroblastoma=== | ===CNS ganglioneuroblastoma=== | ||
Latest revision as of 13:44, 4 October 2017
| Primitive neuroectodermal tumour | |
|---|---|
| Diagnosis in short | |
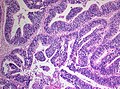
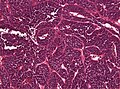
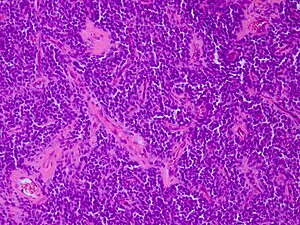 CNS primitive neuroectodermal tumour H&E stain. | |
|
| |
| Synonyms | CNS-PNET |
| LM DDx | small round blue cell tumours |
| IHC | S-100 +ve, Syn +/-ve |
| Site | brain, spinal cord |
|
| |
| Prevalence | rare - typically in young adults |
| Prognosis | poor (WHO Grade IV) |
CNS Primitive neuroectodermal tumour, abbreviated CNS-PNET, is an abandoned neuropathology tumour description within in the group of embryonal tumours.
The terminology was introduced in 1973 [1] and used in the WHO 2007 classification of CNS tumors. Since 2016 this category has been replaced by the designation other CNS embryonal tumors.
General
- Should not be confused with peripheral primitive neuroectodermal tumour (abbreviated pPNET[2]), AKA Ewing sarcoma.
- The former category contained a heterogenous group of poorly differentiated WHO grade IV tumours associated with following ICD-O codes:
- 9473/3 CNS-PNET, NOS.
- 9500/3 CNS neuroblastoma.
- 9490/3 CNS ganglioneuroblastoma.
- 9501/3 Medulloepithelioma.
- 9392/3 Ependymoblastoma.
- Mainly children and adolescents.
- Cerebral hemisphere, brain stem or spinal cord.
- Cerebrospinal dissemination found in up to 1/3 patients.[3]
- Very poor prognosis[4]
Microscopic
Features:
- Small round blue cell tumour.
- Focal differentation into astrocytic, neuronal or ependymal phenotypes possible.
- May have true rosettes (slit-like/oval).
- Growth in streams or palisades possible ("spongioneuroblastoma").
- Vascular endothelial proliferations.
- Fibrillary background in tumours with advanced neuronal maturation (ganglioneuroblastomas).
- Variable mitotic activity.
Supratentorial PNET
- This category of small round- and blue cell tumor was used in the WHO 2007 CNS tumor classification to separate them from medulloblastomas.
- Tumors are today classified as AT/RT, Pineoblastoma, ETMR, H3F3A-mutated glioblastoma or CNS embryonal tumor, NOS.
CNS neuroblastoma
- Since WHO 2016 CNS classification this is now a subgroup of Other CNS embryonal tumours.
- Many CNS neuroblastoma / CNS ganglioneuroblastoma cluster molecularly into a group designated as CNS NB-FOXR2.[5]
- Usu Olig-2 +ve.
- Synaptophysin +ve.
CNS ganglioneuroblastoma
- This is now a subgroup of Other CNS embryonal tumours.
Lipomatous medulloblastoma
- These tumors are now designated as Cerebellar liponeurocytoma.
Melanotic medulloblastoma
- In WHO CNS 1997 still a distinct tumor.
- These tumors are now considered a variant of medulloblastoma.
Medullomyoblastoma
- Embryonal tumor with primitive neuronal cells and striated muscle component.
- First description in 1933.[6]
- Since WHO 2007 CNS tumor classification tumors were classified as medulloblastoma with myogenic differentiation.
Medulloepithelioma
- Neuroepithelial tumor cells arranged papillary, tubular or trabecular.
- Pseudostratified with PAS-positive membrane.
- Medulloepithelioma are grouped with ependymoblastomas and ETANTR into embryonal tumors with multilayered rosettes (ETMR).[7]
- Not the same tumour as the intraocular medulloepithelioma.[8]
Ependymoblastoma
- Often supratentorial, well circumscribed.
- Multilayered ("ependymoblastous") rosettes.
- High mitotic and proliferative activity
- Ependymoblastoma are grouped with medulloepithelioma and ETANTR into embryonal tumors with multilayered rosettes (ETMR).[7]
Immunohistochemistry
- S-100 +ve.
- INI1 +ve (loss defines tumour as ATRT).
- LIN28+ve (in ETMR), otherwise -ve. [9]
- Nestin +ve
- MAP2 +ve/-ve
- Vimentin +ve
- IDH-1 -ve
- No ATRX loss
- MIB-1 between 20-80% (usu. 50%)
Molecular genetics
Divergent molecular subgroups are emerging:
DDx:
- Small round blue cell tumours
- Medulloblastoma
- ATRT (INI1 loss)
- Anaplastic ependymoma (RELA fusions)
- Paediatric glioblastoma (IDH1/2) and (H3F3A mutations)
- Embryonal tumour with abundant neuropil and true rosettes (ETANTR) - currently no distinct WHO entity.[12]
Images
www:
- Primitive neuroectodermal tumour - several images (upmc.edu).
- GBM with PNET component - several images (upmc.edu).
See also
References
- ↑ Hart, MN.; Earle, KM. (Oct 1973). "Primitive neuroectodermal tumors of the brain in children.". Cancer 32 (4): 890-7. PMID 4751919.
- ↑ PST. 14 February 2011.
- ↑ Horten, BC.; Rubinstein, LJ. (Dec 1976). "Primary cerebral neuroblastoma. A clinicopathological study of 35 cases.". Brain 99 (4): 735-56. PMID 1030655.
- ↑ Tulla, M.; Berthold, F.; Graf, N.; Rutkowski, S.; von Schweinitz, D.; Spix, C.; Kaatsch, P. (Sep 2015). "Incidence, Trends, and Survival of Children With Embryonal Tumors.". Pediatrics 136 (3): e623-32. doi:10.1542/peds.2015-0224. PMID 26304823.
- ↑ Sturm, D.; Orr, BA.; Toprak, UH.; Hovestadt, V.; Jones, DTW.; Capper, D.; Sill, M.; Buchhalter, I. et al. (Feb 2016). "New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs.". Cell 164 (5): 1060-1072. doi:10.1016/j.cell.2016.01.015. PMID 26919435.
- ↑ Brody, BS.; German, WJ. (Oct 1933). "Medulloblastoma of the Cerebellum: A Report of 15 Cases.". Yale J Biol Med 6 (1): 19-29. PMID 21433586.
- ↑ 7.0 7.1 Horwitz, M.; Dufour, C.; Leblond, P.; Bourdeaut, F.; Faure-Conter, C.; Bertozzi, AI.; Delisle, MB.; Palenzuela, G. et al. (Oct 2015). "Embryonal tumors with multilayered rosettes in children: the SFCE experience.". Childs Nerv Syst. doi:10.1007/s00381-015-2920-2. PMID 26438544.
- ↑ Korshunov, A.; Jakobiec, FA.; Eberhart, CG.; Hovestadt, V.; Capper, D.; Jones, DT.; Sturm, D.; Stagner, AM. et al. (Jul 2015). "Comparative integrated molecular analysis of intraocular medulloepitheliomas and central nervous system embryonal tumors with multilayered rosettes confirms that they are distinct nosologic entities.". Neuropathology. doi:10.1111/neup.12227. PMID 26183384.
- ↑ Korshunov, A.; Ryzhova, M.; Jones, DT.; Northcott, PA.; van Sluis, P.; Volckmann, R.; Koster, J.; Versteeg, R. et al. (Dec 2012). "LIN28A immunoreactivity is a potent diagnostic marker of embryonal tumor with multilayered rosettes (ETMR).". Acta Neuropathol 124 (6): 875-81. doi:10.1007/s00401-012-1068-3. PMID 23161096.
- ↑ Pfister, S.; Remke, M.; Toedt, G.; Werft, W.; Benner, A.; Mendrzyk, F.; Wittmann, A.; Devens, F. et al. (Sep 2007). "Supratentorial primitive neuroectodermal tumors of the central nervous system frequently harbor deletions of the CDKN2A locus and other genomic aberrations distinct from medulloblastomas.". Genes Chromosomes Cancer 46 (9): 839-51. doi:10.1002/gcc.20471. PMID 17592618.
- ↑ Korshunov, A.; Remke, M.; Gessi, M.; Ryzhova, M.; Hielscher, T.; Witt, H.; Tobias, V.; Buccoliero, AM. et al. (Aug 2010). "Focal genomic amplification at 19q13.42 comprises a powerful diagnostic marker for embryonal tumors with ependymoblastic rosettes.". Acta Neuropathol 120 (2): 253-60. doi:10.1007/s00401-010-0688-8. PMID 20407781.
- ↑ Buccoliero AM, Castiglione F, Degl'Innocenti DR, et al. (February 2010). "Embryonal tumor with abundant neuropil and true rosettes: morphological, immunohistochemical, ultrastructural and molecular study of a case showing features of medulloepithelioma and areas of mesenchymal and epithelial differentiation". Neuropathology 30 (1): 84–91. doi:10.1111/j.1440-1789.2009.01040.x. PMID 19563506.